Chapter 5 Choosing a Quality Control Product
Summary
TLDRChapter 5 emphasizes the importance of selecting quality control products for laboratories, cautioning against the lure of low-cost options that may have limited shelf life and inadequate analyte levels. It advises understanding daily control usage to avoid waste and warns against misleading 'box pricing'. Laboratories should consider the diagnostic utility of products for their test menu, the reliability of results, and the value of additional services like interlaboratory comparison programs. The chapter concludes by stressing the need for accurate, reliable quality control to protect both the lab's reputation and patient health.
Takeaways
- 🧪 Laboratories have various quality control products available, but choosing the right one requires careful consideration.
- 💰 Opting for cheaper quality control products can lead to limitations such as a shorter shelf life and potential waste.
- ⚠️ Some quality control products are not similar enough to patient specimens, which can cause issues with certain test systems.
- 📦 'Box pricing' can be misleading; it's important to ask for quotes on a per mL basis rather than just the box price.
- ⏳ Laboratories should match the quality control product's shelf life with their daily usage to avoid wasting resources.
- 📉 Purchasing quality control products that don't align with the lab's diagnostic criteria can lead to inaccurate test results.
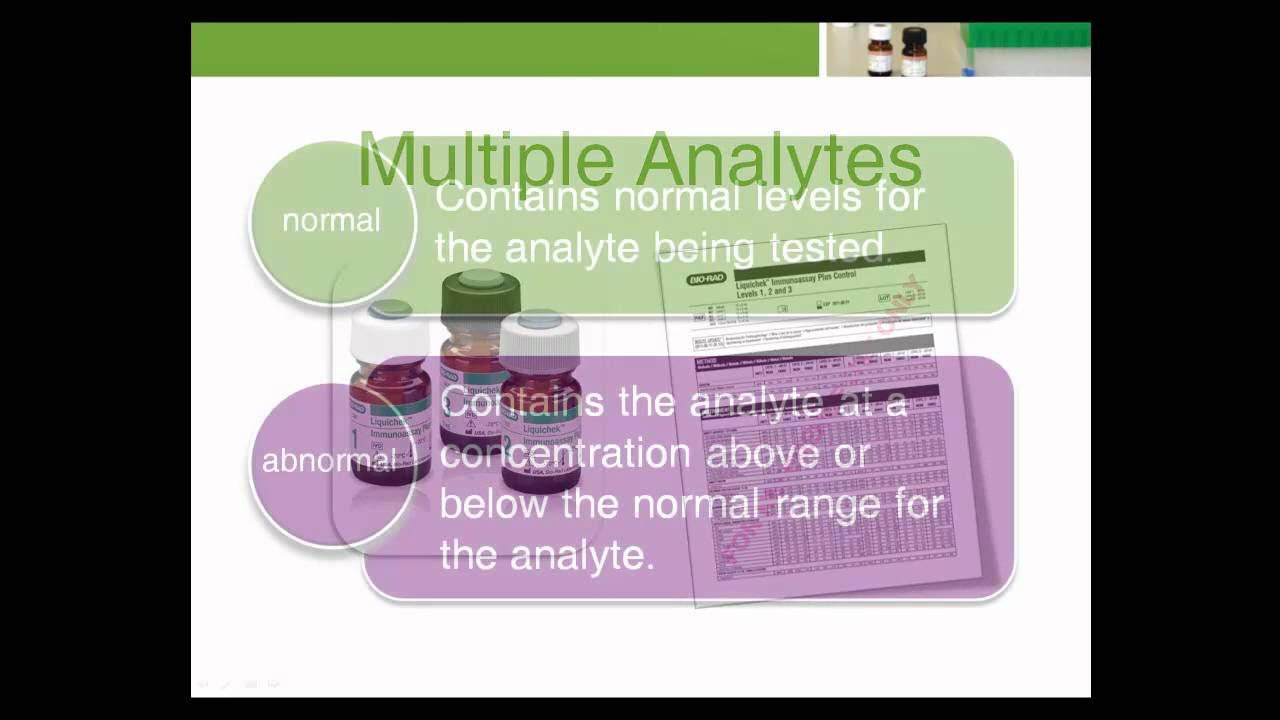
- 🔍 Selecting a quality control product that offers trilevel diagnostic utility is crucial for accurate lab results across different analytes.
- 🏥 Inaccurate lab results can damage a laboratory's reputation and, more importantly, harm patients.
- 📊 Participating in an Interlaboratory Quality Control Comparison Program is recommended to regularly verify the reliability of lab work.
- 🌍 The value of additional services provided by the quality control product manufacturer, such as interlaboratory comparison programs, should be considered when making purchasing decisions.
Q & A
What is the primary consideration when choosing a quality control product for a laboratory?
-The primary consideration is to choose a product that matches the laboratory's needs in terms of stability, similarity to patient specimens, and analyte levels at medically relevant decision points.
Why might a laboratory face unnecessary waste with cheaper quality control products?
-Laboratories might face unnecessary waste because cheaper products often have a shorter shelf life, which can lead to material not being used before it expires if the laboratory's usage rate is low.
How can 'box pricing' mislead laboratory administrators when purchasing quality control products?
-Box pricing can mislead by quoting a price per box without specifying the price per mL, which may seem cheaper but can actually be more expensive when the volume per box is considered.
What is the importance of the shelf life of a quality control product in relation to a laboratory's usage rate?
-The shelf life should match or exceed the laboratory's normal usage rate to prevent waste. If the shelf life is shorter than the time needed to use the product, it can result in significant waste and increased cost per mL.
Why is it crucial for quality control products to have analytes at medically relevant decision levels?
-Having analytes at medically relevant decision levels ensures that the quality control product can adequately challenge the test system and provide accurate control for the full range of patient results.
What is the significance of a quality control product being similar to patient specimens?
-Quality control products need to interact with the test system in a manner similar to patient specimens to ensure accurate and reliable test results.
How can a laboratory assess the value of a quality control product beyond its price?
-A laboratory should assess the product's diagnostic utility across the entire test menu, the vendor's support services, the availability of interlaboratory comparison programs, and the reliability and ISO certification of the vendor.
What are the potential consequences of selecting a quality control product that does not adequately control low or high levels of analytes?
-Selecting a product that does not adequately control analyte levels can lead to incorrect laboratory results, which can damage the laboratory's reputation and, more importantly, harm patients.
Why is participation in an Interlaboratory Quality Control Comparison Program recommended for laboratories?
-Participation in such programs allows laboratories to verify the reliability of their work by comparing their results with other laboratories using the same instrument and method, thus improving overall quality control.
What additional services should a quality control purchaser consider when evaluating a vendor?
-The purchaser should consider whether the vendor provides an interlaboratory comparison program, professional staffing for technical advice, comparative statistical reports, QC software that can import data, educational support, and ISO certification.
Outlines

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنMindmap

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنKeywords

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنHighlights

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنTranscripts

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنتصفح المزيد من مقاطع الفيديو ذات الصلة

Omega 3 Alırken Nelere Dikkat Etmeliyiz? | Omega 3'ün Faydaları Nelerdir?

Chapter 3 Levey-Jennings Charts & Westgard Rules
Chapter 1 Quality Control Basics

Shelf Life of Foods - Definition

THE INTELLIGENT INVESTOR BOOK SUMMARY - CHAPTER 5 - COMMON STOCKS

We Bought Fear of God's $1050 Dry-Clean Sweatpants So You Don't Have To. Teardown and Review
5.0 / 5 (0 votes)
