Mecanismo de substituição aromática eletrofílica | Química orgânica | Khan Academy
Summary
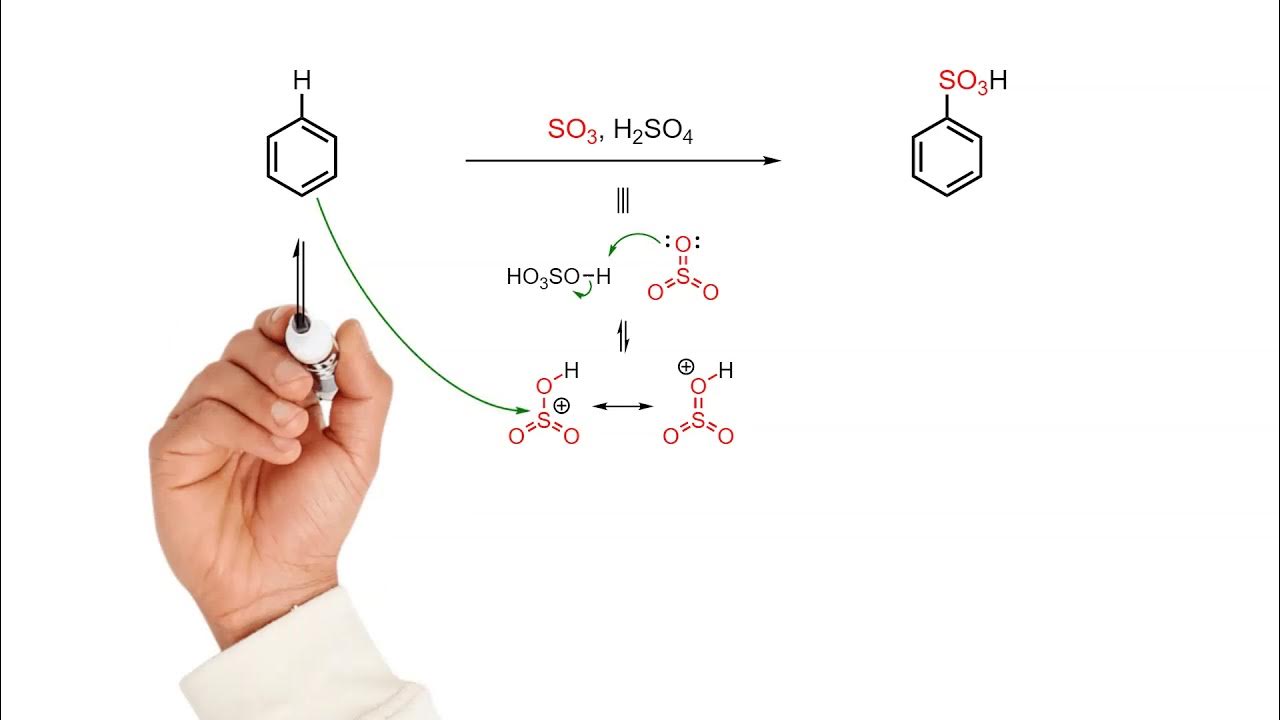
TLDRThis video delves into the electrochemical aromatic substitution reaction, explaining how a benzene ring undergoes proton substitution by an electrolyte, with the help of a catalyst. The process involves the formation of an electrochemical complex, followed by resonance structures that stabilize the reaction intermediate, known as the sigma complex. The mechanism restores the aromatic ring's stability by regenerating the catalyst, allowing for further reactions. Key concepts such as resonance, catalyst interaction, and the electrochemical substitution are thoroughly explored to explain this important organic reaction.
Takeaways
- 😀 Electrochemical aromatic substitution involves replacing a proton in a benzene ring with an electrophile.
- 😀 The aromatic nature of the reaction refers to the regeneration of the aromatic ring during the mechanism.
- 😀 A catalyst is essential to form the electrolyte, which is crucial for the substitution process.
- 😀 The catalyst helps to generate a positively charged electrolyte that is attracted to the benzene ring.
- 😀 The reaction mechanism starts with the interaction between pi electrons in the aromatic ring and the electrophile, forming a covalent bond.
- 😀 A resonance mechanism occurs, where the positive charge moves between carbons in the ring as the reaction progresses.
- 😀 The intermediate formed is a sigma complex, a hybrid of various resonance structures with a delocalized positive charge.
- 😀 The catalyst, once formed, operates as a base to deprotonate the complex, restoring the aromaticity of the ring.
- 😀 The final product of the reaction is the substituted aromatic compound and the catalyst, which is regenerated for further reactions.
- 😀 The proton that is removed from the ring in the final step is paired with the electrophile, forming a new product.
Q & A
What is the main concept behind electrochemical aromatic substitution?
-Electrochemical aromatic substitution is a reaction where one of the protons in an aromatic ring (like benzene) is replaced by an electrophile, forming a new covalent bond while maintaining the aromaticity of the ring.
What role does the catalyst play in the electrochemical aromatic substitution mechanism?
-The catalyst helps to form the electrophile by reacting with the molecule containing the electrophile. It also forms a complex with the electrophile and facilitates the removal of a proton, which restores the aromaticity of the ring.
What happens during the formation of the electrophile?
-The catalyst reacts with a molecule containing the electrophile, forming a positively charged electrophile. This electrophile then interacts with the aromatic ring.
How do the pi electrons in the aromatic ring participate in the reaction?
-The pi electrons in the aromatic ring act as nucleophiles. They are attracted to the positively charged electrophile, and they form a new covalent bond with the electrophile, temporarily breaking the aromaticity.
What is a sigma complex in the context of this reaction?
-A sigma complex is an intermediate structure formed during the reaction. It is a resonance hybrid where the positive charge is delocalized across three carbons in the ring, resulting from the movement of pi electrons.
Why is resonance important in the mechanism of this reaction?
-Resonance is crucial because it allows the positive charge to be delocalized across multiple carbon atoms in the ring. This stabilizes the intermediate sigma complex and facilitates the restoration of the aromatic ring.
What happens during the reformation of the aromatic ring?
-The catalyst acts as a base, extracting a proton from the intermediate sigma complex. This process restores the aromaticity by reforming the double bonds in the ring, removing the positive charge and completing the substitution.
What are the final products of the electrochemical aromatic substitution?
-The final products are the substituted aromatic compound (where the proton is replaced by the electrophile) and a protonated version of the catalyst.
What does it mean that the catalyst is regenerated in this reaction?
-The catalyst is regenerated because, after it extracts a proton from the sigma complex, it is freed to catalyze another reaction. The catalyst is not consumed in the reaction but facilitates the transformation.
How does the reaction maintain the aromaticity of the ring?
-The aromaticity is maintained after the proton is removed, and the double bonds in the ring are reformed through the movement of electrons. The final product retains the stable aromatic ring structure.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级5.0 / 5 (0 votes)