Comportamento Ondulatório da Matéria
Summary
TLDRThis video script discusses the fundamental concepts of modern quantum mechanics, beginning with the duality of light as both a particle and a wave. It explores key developments, including Einstein's contributions to the photoelectric effect and Bohr's atomic model, which led to an understanding of atomic spectra. The script highlights Louis de Broglie's groundbreaking hypothesis, proposing that particles, like electrons, exhibit wave-like behavior. This idea provided a clearer explanation of Bohr’s quantization rule and marked the birth of quantum mechanics. The script also touches on the challenges of observing particle diffraction due to the tiny wavelengths involved.
Takeaways
- 😀 The relationship between the energy of a photon and its frequency demonstrates the duality of light, behaving both as a particle and a wave.
- 😀 The first major advancement in the microscopic theory of matter came with Bohr's atomic model in 1913, which successfully explained the hydrogen spectrum but struggled with more complex atoms.
- 😀 Bohr's model introduced the quantization of angular momentum, but it lacked an explanation for why this quantization exists.
- 😀 In 1923, Louis de Broglie proposed that matter, like light, might exhibit wave-like properties, suggesting a duality for particles as well.
- 😀 De Broglie's hypothesis connected the momentum of a particle to its wavelength and frequency, showing that the same principles applying to light could extend to particles.
- 😀 By associating particles with waves, de Broglie helped explain Bohr's quantization of angular momentum using a new perspective based on wave behavior.
- 😀 The key idea in de Broglie’s theory was that particles could be associated with 'matter waves,' leading to a better understanding of the quantization of electron orbits.
- 😀 De Broglie’s hypothesis justified Bohr's quantization condition by assuming that an electron's orbit would be stable if the associated wave formed a complete circle with an integer number of wavelengths.
- 😀 The proposal that particles have wave-like characteristics helped lay the foundation for modern quantum mechanics, introducing the wave-particle duality concept for matter.
- 😀 Despite its simplicity, de Broglie's idea revolutionized the field, providing a connection between classical and quantum theories, though many questions about the nature of the waves remained unanswered.
- 😀 The wave-like behavior of particles was not experimentally observable before due to their extremely small wavelengths, but electron diffraction experiments eventually confirmed de Broglie’s predictions in 1927.
Q & A
What is the connection between the energy of a photon and its frequency in the script?
-The energy of a photon is related to its frequency through the equation E = h * f, where 'E' is the energy, 'h' is Planck's constant, and 'f' is the frequency. This reflects the wave-particle duality of light.
What is the significance of de Broglie's hypothesis in the development of quantum mechanics?
-De Broglie's hypothesis proposed that matter, like light, also exhibits wave-particle duality. He suggested that particles such as electrons can be associated with a wave, which helped explain the quantization seen in Bohr's atomic model.
How did Einstein’s analysis of the photoelectric effect contribute to the understanding of light as both a particle and a wave?
-Einstein’s analysis of the photoelectric effect showed that light behaves like a particle (photon) when interacting with matter. This supported the idea that radiation can exhibit both wave-like and particle-like properties, a cornerstone of quantum mechanics.
What limitation of Bohr's atomic model is highlighted in the script?
-Bohr's atomic model, while successful in explaining the hydrogen atom spectrum, failed to explain the spectra of more complex atoms or the behavior of multi-electron systems. Additionally, it did not provide a theoretical explanation for the quantization of angular momentum.
What was the major advancement in atomic theory following Bohr’s model?
-The major advancement following Bohr's model was de Broglie’s suggestion that all particles, including electrons, possess a dual wave-particle nature. This led to the development of quantum mechanics, which integrated both wave and particle concepts.
Why was de Broglie’s proposal that particles have associated wavelengths revolutionary?
-De Broglie’s proposal was revolutionary because it extended the wave-particle duality of light to matter, suggesting that even microscopic particles like electrons have wave-like properties. This provided a new framework for understanding atomic and subatomic phenomena.
How did de Broglie’s hypothesis help explain Bohr's quantization condition?
-De Broglie’s hypothesis explained Bohr’s quantization condition by showing that an electron in a stable orbit could be associated with a wave whose wavelength fits perfectly into the orbit. This led to the idea that only certain orbits would allow for constructive interference, justifying the quantization of angular momentum.
What are the implications of de Broglie’s concept of matter waves for experimental physics?
-De Broglie’s concept of matter waves had significant implications for experimental physics, particularly in diffraction and interference experiments. It suggested that particles like electrons could exhibit wave-like behaviors, which were experimentally confirmed in later experiments such as the Davisson-Germer experiment.
Why is it difficult to observe diffraction with low-energy electrons?
-It is difficult to observe diffraction with low-energy electrons because their associated wavelengths are very small, and low-energy electrons are easily scattered by molecules in the air. To observe diffraction, high-energy electrons are needed, and experiments must be conducted in a vacuum to prevent scattering.
What experiment in 1927 confirmed the wave nature of electrons?
-The wave nature of electrons was experimentally confirmed in 1927 by the Davisson-Germer experiment, which observed diffraction patterns produced by electrons, demonstrating that electrons exhibit wave-like behavior.
Outlines

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифMindmap

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифKeywords

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифHighlights

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифTranscripts

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифПосмотреть больше похожих видео

The Quantum Theory of the Atom | Chapter 7 - General, Organic, and Biological Chemistry

Does CONSCIOUSNESS Create REALITY According To Quantum Mechanics?

Menjawab Misteri Besar Fisika: Apakah Cahaya Gelombang atau Partikel?

Particles and waves: The central mystery of quantum mechanics - Chad Orzel
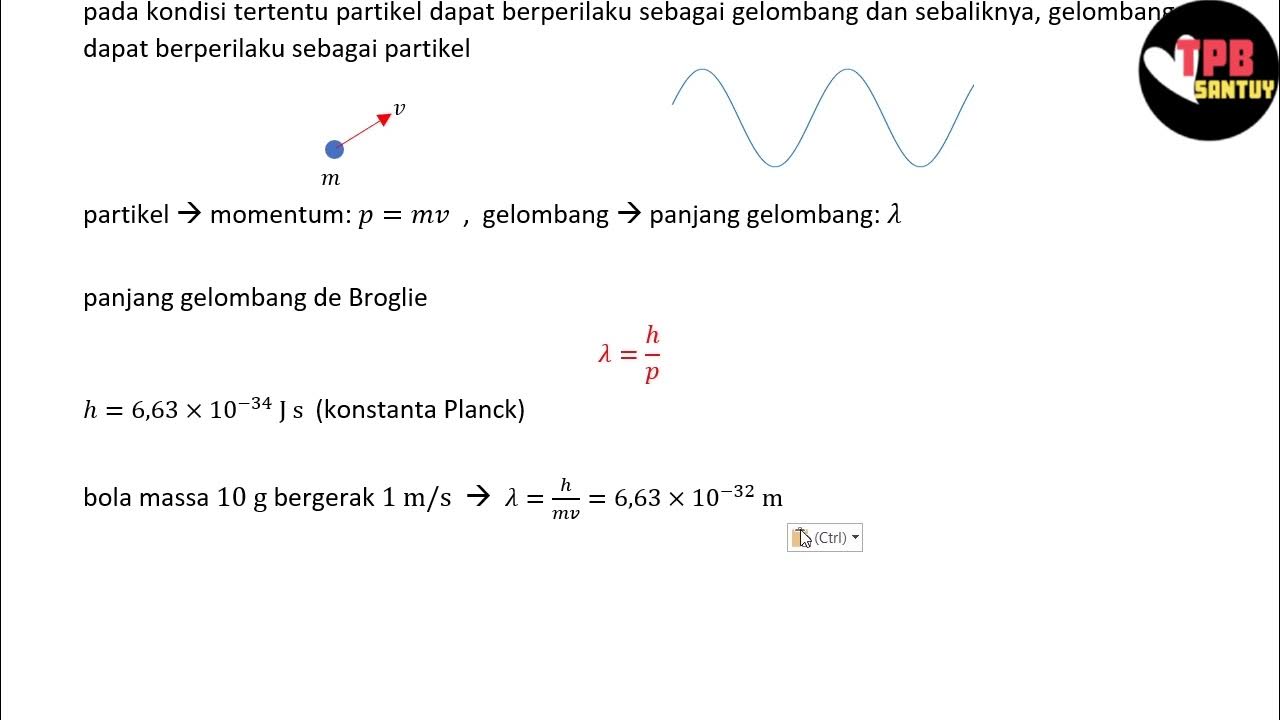
Dualisme Gelombang-Partikel | Fenomena Kuantum | Part 1 | Fisika Dasar

The Double-Slit Experiment
5.0 / 5 (0 votes)
